An In-Depth Guide to Goal-Directed Fluid Therapy (GDFT)
1. What is Goal-Directed Fluid Therapy (GDFT)?
Goal-Directed Fluid Therapy (GDFT), also known as goal-directed therapy or hemodynamic optimization, is a modern, evidence-based approach to intravenous fluid administration. Instead of relying on traditional, often static parameters, GDFT uses dynamic, real-time hemodynamic monitoring to guide fluid therapy with the specific aim of optimizing a patient's cardiovascular status and tissue oxygen delivery.
In essence, GDFT is a shift away from a "one-size-fits-all" or "give fluids until the pressure looks good" mentality to a personalized, physiologically-driven strategy. The core principle is to answer two critical questions before giving a fluid bolus:
- Does this patient need more fluid? (Are they fluid responsive?)
- If I give fluid, will it improve their cardiac output and thus oxygen delivery to the tissues?
The "goal" in GDFT is typically to maximize Stroke Volume (SV) or Cardiac Output (CO), which are the primary determinants of tissue perfusion, without causing fluid overload.
2. What is the Need for GDFT? (The Rationale)
The need for GDFT arises from the significant dangers associated with both under-resuscitation and over-resuscitation with fluids. Traditional fluid therapy often walks a tightrope between these two extremes, frequently falling off on one side or the other.
A. The Dangers of Under-Resuscitation (Hypovolemia):
- Reduced Preload: Insufficient blood volume returning to the heart.
- Decreased Stroke Volume & Cardiac Output: The heart has less to pump, leading to low blood pressure.
- Organ Hypoperfusion: Inadequate blood flow leads to a lack of oxygen and nutrients to vital organs.
- Consequences: This can result in Acute Kidney Injury (AKI), gut ischemia (which can lead to bacterial translocation and sepsis), lactic acidosis, and multi-organ dysfunction.
B. The Dangers of Over-Resuscitation (Fluid Overload):
Fluid is a drug, and like any drug, it has a toxicity profile. Giving too much fluid can be just as harmful, if not more so, than giving too little.
- Increased Tissue Edema: Fluid leaks from the intravascular space into the interstitial space.
- Pulmonary Edema: Fluid in the lungs impairs gas exchange, leading to hypoxia and respiratory failure.
- Gut Edema: Swelling of the intestinal wall can impair function and perfusion.
- Impaired Oxygen Diffusion: Increased distance between capillaries and cells makes it harder for oxygen to reach tissues.
- Coagulopathy & Dilutional Anemia: Dilution of clotting factors and red blood cells.
- Delayed Wound Healing & Increased Risk of Infection: Edematous tissues are more prone to poor healing and infection.
- Increased Morbidity and Mortality: Numerous studies have linked positive fluid balance to worse outcomes in surgical and critically ill patients.
The "Sweet Spot": GDFT is designed to find the physiological "sweet spot" on the Frank-Starling curve—the point where the heart is pumping most efficiently without being overloaded. It aims to keep the patient on the ascending, steep part of the curve, where small increases in preload lead to significant increases in stroke volume.
3. How Do We Monitor for GDFT?
The key to GDFT is using the right tools. Monitoring is divided into static and dynamic parameters.
A. Static Parameters (The Old Way - Poor Predictors of Fluid Responsiveness) These are single-point measurements and do not tell us how the cardiovascular system will respond to a change in volume.
- Central Venous Pressure (CVP): The pressure inside the thoracic vena cava. For decades, a CVP of 8-12 mmHg was considered "optimal." We now know CVP is a poor indicator of volume status and does not predict fluid responsiveness. A high CVP can mean volume overload, but it can also mean right ventricular dysfunction or high intrathoracic pressure.
- Blood Pressure (BP): A late indicator of shock. The body compensates for significant volume loss before blood pressure drops.
- Heart Rate (HR): Non-specific and influenced by many factors (pain, fever, medications).
- Urine Output: A useful marker of renal perfusion but is a lagging indicator and can be affected by diuretics or renal dysfunction.
B. Dynamic Parameters (The GDFT Way - Good Predictors of Fluid Responsiveness) These parameters assess the change in stroke volume or its surrogates in response to a physiological challenge, like a breath. They tell us if the patient is on the steep or flat part of the Frank-Starling curve.
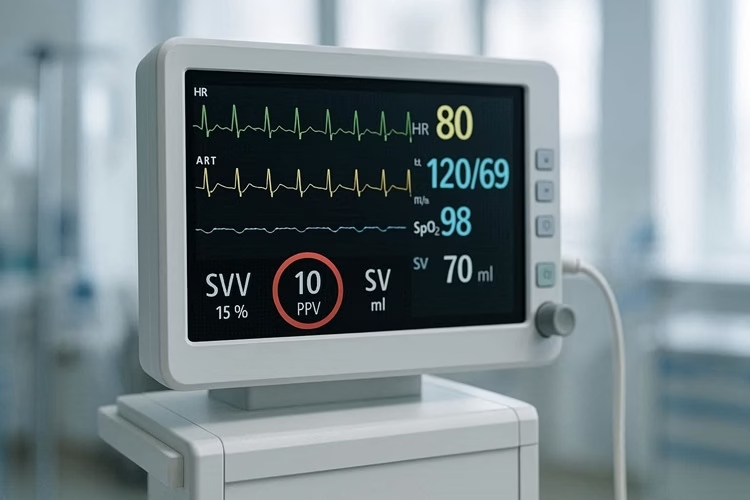
a〉 Stroke Volume Variation (SVV) & Pulse Pressure Variation (PPV)
- How it works: In patients on controlled mechanical ventilation, the positive pressure breath increases intrathoracic pressure, temporarily decreasing venous return (preload) and thus stroke volume. The magnitude of this swing with each breath reflects how sensitive the heart is to preload changes.
- Interpretation:
- High Variation (>10-15%): The patient is fluid responsive (on the steep part of the curve). A fluid bolus will likely increase CO.
- Low Variation (<10%): The patient is likely not fluid responsive (on the flat part of the curve). More fluid will not help and may cause harm.
- Limitations: Only valid in patients who are fully sedated, on controlled ventilation with a regular sinus rhythm, and have normal tidal volumes (8 ml/kg).
b〉 Esophageal Doppler Monitoring (EDM)
- How it works: A thin ultrasound probe is placed in the patient's esophagus, where it lies adjacent to the descending aorta. It measures blood flow velocity in the aorta with each heartbeat, from which it calculates Stroke Volume (SV) and Cardiac Output (CO) in real-time.
- How it's used in GDFT: A fluid challenge is given. If SV increases by >10%, the patient is a responder and another bolus may be given. The goal is to maximize SV.
c〉 Pulse Contour Analysis (e.g., PiCCO, LiDCO)
- How it works: These systems analyze the arterial waveform from an arterial line to continuously calculate SV and CO. They can also provide other useful parameters like SVV.
- How it's used: Similar to EDM, they are used to track changes in SV in response to a fluid challenge to guide therapy.
d〉 Echocardiography (Point-of-Care Ultrasound - POCUS)
- How it works: Ultrasound provides a direct look at the heart and major vessels.
- Inferior Vena Cava (IVC) Collapsibility/Distensibility: In spontaneously breathing patients, a collapsible IVC (>50%) suggests hypovolemia. In ventilated patients, a distensible IVC (>18%) suggests fluid responsiveness.
- LVOT VTI (Left Ventricular Outflow Tract Velocity Time Integral): A surrogate for stroke volume. A change in VTI after a Passive Leg Raise (see below) or fluid challenge can assess fluid responsiveness.
- Visual Assessment: Directly visualize ventricular size and contractility to rule out heart failure as a cause of hypotension.
e〉 The Passive Leg Raise (PLR) Test
- How it works: This is a simple, reversible "auto-transfusion." By elevating the patient's legs to 45°, you gravity-shift approximately 250-300 ml of blood from the lower limbs to the central circulation. This mimics a fluid challenge without actually giving any fluid.
- How it's used: You monitor a hemodynamic parameter (like SV, CO, or even just arterial pulse pressure) before and during the PLR.
- Responder: An increase in SV/CO of >10% during PLR predicts fluid responsiveness.
- Non-responder: No significant change means the patient will not benefit from fluids.
- Advantages: Works in spontaneously breathing patients and those with arrhythmias.
4. How Do We Achieve GDFT? (The Algorithm)
Achieving GDFT involves a structured protocol or algorithm. While specifics vary by institution and monitoring device, the core steps are the same.
A Typical GDFT Algorithm:
- Initial Assessment: Identify a high-risk patient (e.g., major surgery, sepsis, trauma) who could benefit from hemodynamic optimization.
- Baseline Measurement: Establish a baseline for your chosen dynamic parameter (e.g., SVV, SV via EDM, or perform a PLR test).
- The Decision Point: Is the Patient Fluid Responsive?
- If YES (e.g., SVV > 15%, PLR positive), proceed to Step 4.
- If NO (e.g., SVV < 10%, PLR negative), STOP giving fluids. Consider other causes of hypotension, such as vasodilation (need a vasopressor like norepinephrine) or cardiac dysfunction (need an inotrope like dobutamine).
- The Fluid Challenge: Administer a standardized bolus of fluid (e.g., 250 ml of a crystalloid like Hartmann's or Lactated Ringer's) over a short period (e.g., 10-15 minutes). A "mini-bolus" of 100 ml can also be used.
- Re-evaluation: Re-measure your target parameter (e.g., Stroke Volume).
- Responder: If Stroke Volume increased by >10-15%, the patient has benefited. You can either:
- Give another fluid bolus if the patient is still deemed hypovolemic and the goal has not been met.
- Stop if the goal (e.g., optimized SV) has been reached.
- Non-responder: If Stroke Volume did not increase significantly, the patient is now on the flat part of the Frank-Starling curve. STOP giving fluids. The patient is now "fluid optimized."
- Responder: If Stroke Volume increased by >10-15%, the patient has benefited. You can either:
- Optimize Other Parameters: If the patient is fluid optimized but still has signs of poor perfusion (e.g., high lactate, low ScvO2), the next step is to use inotropes (to improve the heart's contractility) or vasopressors (to improve vascular tone), guided by further hemodynamic assessment.
The "Goal": The goal is not just a number. It's the clinical state of adequate tissue oxygen delivery, which is confirmed by:
- Optimized and stable Stroke Volume.
- Stabilization or improvement in lactate levels.
- Adequate urine output (>0.5 ml/kg/hr).
- Good peripheral perfusion (warm, well-perfused skin).
5. Benefits of GDFT:
- Reduced Postoperative Complications: Fewer cases of AKI, respiratory failure, wound infections, and cardiovascular events.
- Shorter Hospital Stay: Patients recover faster and are discharged sooner.
- Reduced Mortality: Significant mortality reduction has been demonstrated in high-risk surgical patients and in some subsets of septic patients when GDFT is applied early.
- Cost-Effectiveness: Despite the cost of monitoring equipment, the reduction in complications and length of stay makes GDFT a cost-effective strategy.
6. Limitations and Challenges:
- Equipment and Training: Requires access to specialized monitoring devices and, crucially, staff who are trained to use and interpret them correctly.
- Patient Limitations: As mentioned, dynamic parameters like SVV/PPV are not valid in all patients (e.g., spontaneous breathing, arrhythmias, low lung compliance).
- Protocol Adherence: The success of GDFT depends on clinicians following the protocol consistently. "Protocol fatigue" can be an issue.
- Not a Panacea: GDFT optimizes fluid status, but it doesn't fix the underlying disease (e.g., uncontrolled sepsis, severe cardiac dysfunction).
7. Clinical Applications:
- Perioperative Medicine: The most established application. Used for high-risk surgeries like major abdominal, orthopedic, and vascular procedures.
- Intensive Care Unit (ICU): Used in the management of septic shock, ARDS (to avoid fluid overload), and other states of circulatory shock.
- Emergency Department: Early GDFT in septic patients (as part of early goal-directed therapy) has been studied, though protocols have evolved.
8. The Future of GDFT:
- Automation: Closed-loop systems where a computer algorithm automatically administers fluid based on real-time SVV data are being developed and tested.
- Integration with AI: Artificial intelligence could help integrate multiple data streams (hemodynamic, lab values, vital signs) to provide more sophisticated treatment recommendations.
- Wider Adoption of POCUS: As ultrasound skills become more common among frontline physicians, POCUS will likely play an even larger role in bringing GDFT principles to a wider patient population.
Conclusion
Goal-Directed Fluid Therapy represents a fundamental paradigm shift in fluid management. By moving away from crude, static pressure-based targets and embracing dynamic, physiologically-guided assessment, GDFT allows clinicians to personalize fluid therapy for each patient. This approach successfully navigates the narrow path between the dangers of hypovolemia and fluid overload, leading to demonstrably better outcomes, fewer complications, and a more efficient use of healthcare resources. It is a cornerstone of modern perioperative and critical care medicine.